Why the PASHiOn Trial?
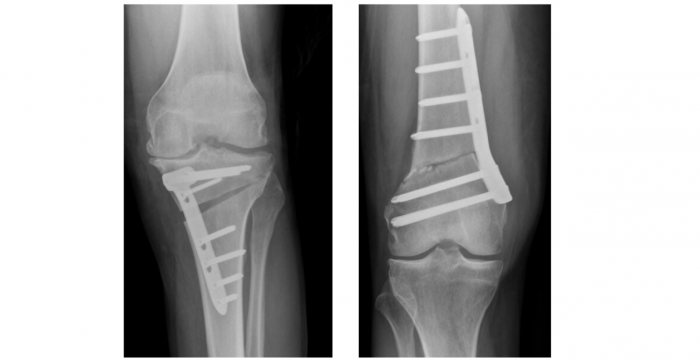
Knee osteoarthritis (OA) is common, painful, progressive and disabling, and has significant personal and societal burden, particularly in an ageing population. Whilst knee replacement is successful for very late stage disease, it is inappropriate for earlier stages leaving few effective treatments available for patients in the “treatment gap” between symptom free and late-stage arthritis. High Tibial Osteotomy (HTO) is a proven surgical treatment providing good long-term outcome, reducing pain and improving function. It is suitable for early to mid-stage knee arthritis and has enormous potential to fill the “treatment gap” for many knee arthritis sufferers.
HTO provides an effective for these patients by correcting the alignment of the knee joint loading and therefore modifying the disease progression. A new method (ToKa) has been devised involving personalisation and digital planning. This method uses a custom personalised surgical guide and plate which potentially overcome existing technical limitations.
The aim of the PASHiOn Trial is to assess whether, in patients with early to mid-stage osteoarthritis of the knee, a personalised digitally planned HTO with custom 3D printed patient specific surgical guides and plate produce a more accurate correction and results in better outcomes compared to standard HTO performed with a “one-size fits all” plate.